Alexia Blesius, PharmD, MSc is Director, Development Consulting and Scientific Affairs
Elisabeth Rouits, PharmD, PhD is Senior Director, Head of Clinical Pharmacology and Translational Medicine in Development Consulting and Scientific Affairs
The early clinical development stage is intrinsically linked to uncertainty regarding both the possible benefits and risks of a novel drug candidate for clinical trial participants.
In its guideline on risk mitigation for first-in-human and early clinical trials, the European Medicines Agency (EMA) notes: “Strategies for development … should always be science-based and decisions should be based on a rigorous interpretation of the totality of the available data[1]”
Before clinical trials can get underway, developers must determine a safe starting dose with relevant dose-escalation plans for their first-in-human (FIH) trial. This is a challenge, since the tested dose-levels should be low enough to avoid unacceptable adverse reactions in the FIH participants while high enough to encompass the biological effects of the potential therapeutic dose range. One approach that has proven to be invaluable in support of drug development strategy, including dose selection, is the Pharmacological Audit Trail (PhAT)[2].
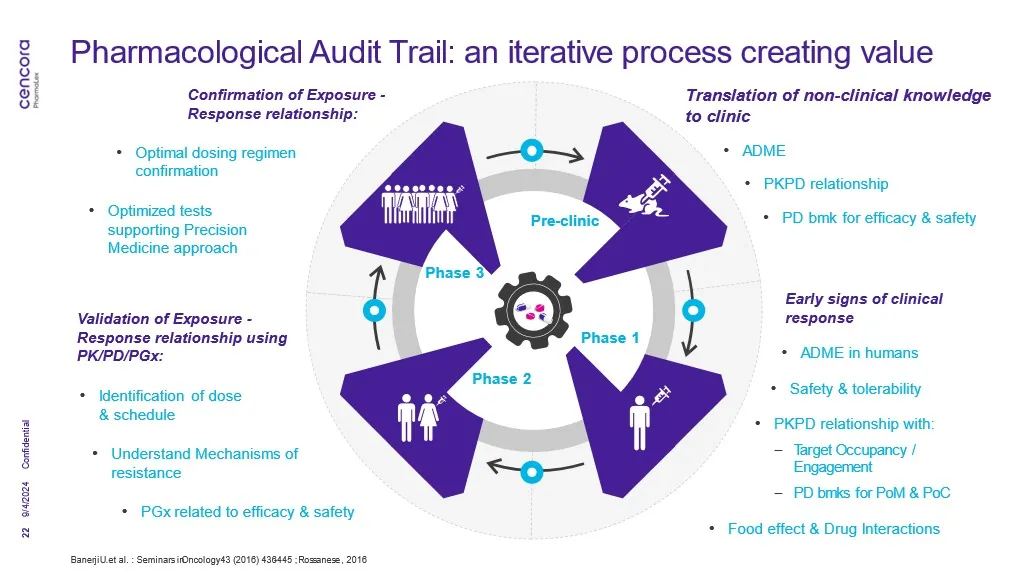
Initially established as a concept and gold-standard framework for developing new precision therapies in oncology, the PhAT relies on evidence-based decision-making and comprises a series of key questions that support drug development strategy[3].
“The PhAT is based on addressing essential questions relating to biomarkers at the appropriate stages of drug development, aiming to maximize our chances of success,” the developers of PhAT framework stated2. These essential questions are:
- Define the patient population
- Establish pharmacokinetic (PK) characteristics
- Provide evidence of target engagement, pathway modulation and biological effects with proof-of-concept (PoC) pharmacodynamic (PD) biomarkers
- Determine intermediate biomarker response
- Assess drug response
- Assess how to overcome drug resistance through combination or sequential therapy
Ref: The figure above is adapted from the PhAT process laid out by experts from the literature referenced in this blog
While developed with anticancer drugs in mind3, in our experience, the PhAT is also a valuable approach in the development of any new drug candidate.
It starts by using a modelling approach that integrates all nonclinical data available before FIH (i.e., in vivo or ex vivo animal studies or in vitro studies) to build knowledge about the drug efficacy and safety profiles in humans. Specifically, mathematical models are built to characterize the relationship between the drug PK – in other words, exposure to the drug – and the drug PD – its biological effect via the assessment of relevant biomarkers of efficacy and/or safety. The development of those PK and/or PK/PD models will support the estimation of equivalent exposures and biological effects in the FIH participants[4].
The PhAT is described as a biomarker-driven roadmap from the laboratory to the clinic to track the effectiveness of drugs for evidence-based decision-making during drug discovery and development3,4.
Once you have the first clinical data, the idea is to enrich and adapt the existing PK and PK/PD models to make them more and more predictive of further clinical outcomes, which in turn supports decisions about the next steps in your clinical development (e.g., PoC or phase 2 study design, etc.). Indeed, from our experience, PhAT is an iterative, data-driven approach where any new emerging clinical data are used to enrich the models and simulate the clinical PD responses to treatment for the tested therapeutic dose range.
A PhAT framework is a massive undertaking that combines sophisticated PK/PD mathematical models and deep biological insight that are key to understanding the drug efficacy and safety profiles. By helping to determine the most relevant biomarkers (of safety and/or efficacy) for each development stage, PhAT ensures a secure linkage between the different drug development stages and maximizes its probability of success.
Finally, knowledge generated using a PhAT approach allows developers to understand how the drug acts on a specific biological pathway, thereby enabling an evidence-based determination about whether it might be effective in another indication.
Achieving regulatory goals
In addition to enhancing the drug development process, adopting a well-defined framework such as the PhAT is widely encouraged by the regulators and complies with their recommendations.
For example, the US Food and Drug Administration (FDA) established Project Optimus with the goal of helping drug developers to reform dose optimization and dose selection in oncology drug development[5]. Specifically, Project Optimus is focused on the development of strategies for dose optimization that takes into account nonclinical and clinical data in dose selection – a goal that is also reflected in the PhAT.
“An emphasis of such strategies will be placed on performing these studies [FIH and early clinical trials] as early as possible in the development program and as efficiently as possible to bring promising new therapies to patients,” the FDA stated5, underscoring the importance of starting a well-defined dose selection process as early as possible, before FIH trials, to inform drug development.
In addition, the FDA has announced a Model-Informed Drug Development (MIDD) Paired Meeting Program to further advance the development and application of “exposure-based, biological, and statistical models derived from preclinical and clinical data sources in drug development and regulatory review. Such an approach has the potential to optimize drug dosing and improve the probability of regulatory success.
The European Medicines Agency (EMA) is also keenly focused on strategies to improve decisions around dosing, noting in its guidelines on risk mitigation for FIH trials, that in the context of FIH and early clinical trials “data generated during a trial should also be used to inform the decision processes for the continuation of dosing. In those cases where an integrated protocol is used, the data generated during the trial should also be used to inform the decision to initiate a subsequent study part (e.g. MAD [Multiple Ascending Dose] or food-effect component), or to inform the selection of the doses of IMP [Investigational Medicinal Product] to be evaluated for components being conducted sequentially or in overlapping fashion, respectively.1”
Achieving the objectives of the regulatory authorities while also streamlining and improving the drug development process – starting in preclinical stage– is an important goal for industry. Through the PhAT framework, developers can take advantage of an iterative, evidence-based approach to safe and effective drug development.
About the authors:
Alexia Blesius, PharmD, MSc is Director, Development Consulting and Scientific Affairs, at PharmaLex. She has 20 years of experience in R&D pharma company acting first as clinical pharmacologist and then as asset leader of several drug development programs from preclinical stage up to registration at global level.
Elisabeth Rouits, PharmD, PhD is Senior Director, Head of Clinical Pharmacology and Translational Medicine in Development Consulting and Scientific Affairs, at PharmaLex. She has more than 20 years of experience in drug development in academia and R&D pharma companies. First acting as clinical pharmacologist for personalized therapies in hospital cancer centers, she moved to a clinical pharmacology strategic lead role mainly in oncology (covering translational to registration milestones) in different pharma companies.
[1] Guideline on strategies to identify and mitigate risks for first-in-human and early clinical trials with investigational medicinal products, EMA, 2017. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-strategies-identify-and-mitigate-risks-first-human-and-early-clinical-trials-investigational-medicinal-products-revision-1_en.pdf
[2] Critical parameters in targeted drug development: the pharmacological audit trail, Seminars in Oncology, 2016. https://www.sciencedirect.com/science/article/pii/S0093775416300306
[3] Developing the Pharmacological Audit Trail. https://www.icr.ac.uk/about-us/our-achievements/our-scientific-discoveries/developing-the-pharmacological-audit-trail#:~:text=The%20pharmacological%20audit%20trail%20(PhAT)%3A%20Use%20of%20tumor%20models,development%20of%20targeted%20anticancer%20drugs
[4] The pharmacological audit trail (PhAT): Use of tumor models to address critical issues in the preclinical development of targeted anticancer drugs, Drug Discovery Today: Disease Models, 2016. https://www.sciencedirect.com/science/article/pii/S1740675717300270
[5] Project Optimus, FDA. https://www.fda.gov/about-fda/oncology-center-excellence/project-optimus